
|
Lifecare Medical Equipments Co., Ltd.
|
test boxes
| Payment Terms: | T/T |
| Place of Origin: | Zhejiang, China (Mainland) |
|
|
|
| Add to My Favorites | |
| HiSupplier Escrow |
Product Detail
It is a new generation of medicine stability test equipment with years of designing and production experience of the company andwith imported German t
It is a new generation of medicine stability test equipment with years of designing and production experience of the company andwith imported German technology. It has broken through the defect that the existing stable temperature and humidity box is unable to operate in succession for a long time. All main parts are imported high-grade products. It has the characteristics of stable, safe and reliable.
Summary:
Creating a long time environment of stable temperature, humidity and light suitable for test of medicine effectiveness through scientific method is applicable for medicine manufacturing companies in long time test, high temperature test, and strong light test for medicine and new medicine. It is the best choice for medicine stability test for medicine manufacturing companies.
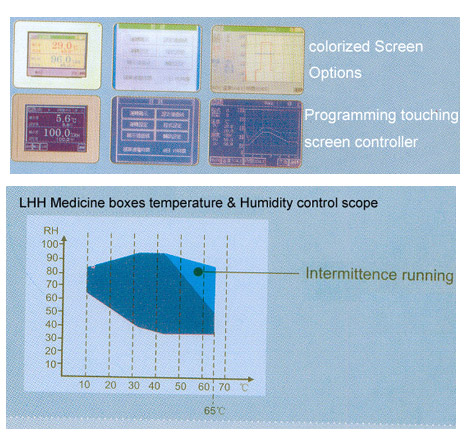
Programming touching screen controller:
※With super huge screen touching style interface, it is easy to operate and the writing of the program is easy.
※There are English and Chinese operating interface for selection. The real-time run curve can be displayed on the screen.
※It has the capacity of 100 groups program, 1000 phrases 999 circulating steps. The max for each time part is 99 hours and 59 minutes.
※After the material and test conditions are input, the controller can lock the screen to avoid miss operating.
※It has P.I.D. automatic figuring function and it can modify temperature and humidity conditions immediately.
※It has RS-232 or RS-485 communication interface. You can design program through computer, monitor test procedure and execute the function of starting and stopping the machine.
Features:
1. With microcomputer to control temperature, humidity, the controlling is stable, acute and reliable.
2. With unique wind circulation system, it is ensured that the wind inside the working room is distributed evenly.
3. With two sets of full closed French imported compressing machine exchanging automatically, the equipment can work continuously for long time.
4. The key parts such as wet temperature controller, compressing machine, circulating fan and son on are all imported products and they are stable, safe and reliable.
5. With isolated over-temperature, low temperature light and sonic track and alarming system, it can ensure your test work safely without any accidents.
6. Temperature increasing and decreasing system, humidity increasing system are all fully isolated so that the work efficiency is improved.
7. With imported stainless steel inner part, it is easy to be cleaned.
8. There is a test hole (dia 50mm) on the left side of the box and there are standard socket for recorder.
Satisfying the standards of : 2000 edition Medicine Stability Test Guideline and relative items in GB 10586-89
Stability test conditions:
In ICH guideline, in aspects of function, performance, and life, GMP & FDA define the requests. Europe. Japan and USA are agreed to make a general stability test. And the purpose of these tests is to collect information to make a recommendation for the stability of medicine or for raw material. The last purpose is prove the effectiveness of the medicine exposed in an environment with a certain temperature, humidity and light in a certain period.
The strorage conditions for stability test samples that will be kept for a long time:
Temperature: +30 ℃± 2 ℃
Humidity: 60 ± 5%RH
Time: 12 months
Storage conditions for accelerated stability test sample:
Temperature: +40 ℃± 2 ℃
Humidity: 75 ± 5%RH
Time: 6 months
Strong light irradiation condition:
Illumination: 4500 ± 500LX
※Above data are for reference only.
Special technical measure:
1. Refrigeration system: Two sets of originally imported refrigeration system and they can exchange automatically to ensure that the equipment can work for a long time continously.
2. Adjusting method: Wit balanced temperature and humidity adjusting method, it can ensure the temperature and humidity are accurate and reliable with small fluctuation.
3. Safety protection: It has isolated temperature limit protection system.
4. Data processing: it has real time data printing and recording system.
Summary:
Creating a long time environment of stable temperature, humidity and light suitable for test of medicine effectiveness through scientific method is applicable for medicine manufacturing companies in long time test, high temperature test, and strong light test for medicine and new medicine. It is the best choice for medicine stability test for medicine manufacturing companies.
Programming touching screen controller:
※With super huge screen touching style interface, it is easy to operate and the writing of the program is easy.
※There are English and Chinese operating interface for selection. The real-time run curve can be displayed on the screen.
※It has the capacity of 100 groups program, 1000 phrases 999 circulating steps. The max for each time part is 99 hours and 59 minutes.
※After the material and test conditions are input, the controller can lock the screen to avoid miss operating.
※It has P.I.D. automatic figuring function and it can modify temperature and humidity conditions immediately.
※It has RS-232 or RS-485 communication interface. You can design program through computer, monitor test procedure and execute the function of starting and stopping the machine.
Features:
1. With microcomputer to control temperature, humidity, the controlling is stable, acute and reliable.
2. With unique wind circulation system, it is ensured that the wind inside the working room is distributed evenly.
3. With two sets of full closed French imported compressing machine exchanging automatically, the equipment can work continuously for long time.
4. The key parts such as wet temperature controller, compressing machine, circulating fan and son on are all imported products and they are stable, safe and reliable.
5. With isolated over-temperature, low temperature light and sonic track and alarming system, it can ensure your test work safely without any accidents.
6. Temperature increasing and decreasing system, humidity increasing system are all fully isolated so that the work efficiency is improved.
7. With imported stainless steel inner part, it is easy to be cleaned.
8. There is a test hole (dia 50mm) on the left side of the box and there are standard socket for recorder.
Satisfying the standards of : 2000 edition Medicine Stability Test Guideline and relative items in GB 10586-89
Stability test conditions:
In ICH guideline, in aspects of function, performance, and life, GMP & FDA define the requests. Europe. Japan and USA are agreed to make a general stability test. And the purpose of these tests is to collect information to make a recommendation for the stability of medicine or for raw material. The last purpose is prove the effectiveness of the medicine exposed in an environment with a certain temperature, humidity and light in a certain period.
The strorage conditions for stability test samples that will be kept for a long time:
Temperature: +30 ℃± 2 ℃
Humidity: 60 ± 5%RH
Time: 12 months
Storage conditions for accelerated stability test sample:
Temperature: +40 ℃± 2 ℃
Humidity: 75 ± 5%RH
Time: 6 months
Strong light irradiation condition:
Illumination: 4500 ± 500LX
※Above data are for reference only.
Special technical measure:
1. Refrigeration system: Two sets of originally imported refrigeration system and they can exchange automatically to ensure that the equipment can work for a long time continously.
2. Adjusting method: Wit balanced temperature and humidity adjusting method, it can ensure the temperature and humidity are accurate and reliable with small fluctuation.
3. Safety protection: It has isolated temperature limit protection system.
4. Data processing: it has real time data printing and recording system.

Didn't find what you're looking for?
Post Buying Lead or contact
HiSupplier Customer Service Center
for help!





